40 eu language requirements for product labels
Product Labeling Requirements: What You Need To Know The US, Canada, Mexico, and the EU all require that your product packaging be written in local languages. In Canada, that means your labels need to be in English and French. In Mexico, that means Spanish. Choosing a Labeling Translation and Compliance Partner European Language Translation Requirements for Medical Device Labeling ... Translations aren't limited to your product labeling and instructions for use. MDR Article 19(1) makes that clear: "The EU declaration of conformity shall state that the requirements specified in this Regulation have been fulfilled in relation to the device that is covered. The manufacturer shall continuously update the EU declaration of ...
Product Labelling — EUbusiness.com | EU news, business and politics Almost all products sold within the EU will require some form of labeling, but what details are required depends on the nature of the product. Here are a few examples of the topics covered by EU legislation: EU Food Product Labeling; General legislation covering labeling, presentation and advertising Nutritional Alcoholic beverages

Eu language requirements for product labels
Official Language in EU required for Medical Device Labeling Medical Device Label - Language: EU Medical Device Regulations: 2: May 11, 2022: L: Language of quality system in case of FDA inspection: US Food and Drug Administration (FDA) 1: Feb 3, 2022: B: Software as a Medical Device - Language Requirements: EU Medical Device Regulations: 6: Jan 21, 2022: Q: World-wide language requirements: Other ... European Union Product Labeling Requirements: A Complete Guide Below follows criteria for Textiles and Furniture: Textiles 1. The product shall not contain lead-based pigments. 2. Manufacturers shall perform colorfastness, washing, wet rubbing, dry rubbing tests on dyed yarn, final fabrics, or final products. 3. Manufactured elastane shall not contain organotin compounds Furniture 1. EU Labeling - Your Consultant on European Regulation Only once the EU compliance process has been almost completed (including the safety tests, safety assessment) will a product's label be verified and the requirements applied in reference to the specifics of the product. Scope & Classification. The Regulation: EC 1223/2009. EU Responsible Person. Product Information File.
Eu language requirements for product labels. United States Product Labeling Requirements: An Overview Bag Suffocation Warning labeling is required when selling products in certain types of plastic bags on Amazon, and also a legal requirement in California, New York, and other states. Note that the specific warning labeling requirements (e.g. the text printed on the plastic bag) differ depending on the following factors: EU Labeling Requirements - United States Mission to the European Union EU Labeling Requirements General labeling requirements The standard U.S. label does not comply with the EU's labeling requirements. On December 13, 2014, the EU's new "Food Information to Consumers (FIC)" Regulation 1169/2011becomes applicable and introduces new obligations and changes to the existing rules. EU - Labeling/Marking Requirements Starting on July 16, 2021, all CE marked products will need to have an EU address on the label. This also applies to products sold online. The name and address must appear on the product or the product's packaging so that customs and market surveillance authorities can have a contact person in case the product is suspected to present a risk. EU Language Requirements | Obelis The table below provides an overview of the official languages in the EU Member States with the aim to assist the manufacturer to know which languages should appear on the labels of cosmetic products to be place on specific markets.
How to Create a Label as per EU MDR 2017/745? Innovations by MDR 2017/745. Manufacturers of medical devices are subject to a number of new requirements as a result of the EU Medical Device Directive, mainly the following: The scope of application also extends to non-medical products (e.g. contact lenses, devices for liposuction). Each medical device must bear a unique identification number ... EU MDR language requirements - Decomplix A glance at the MDR shows that Article 10 (11) very fundamentally requires manufacturers to include information in one or more official language (s) determined by the Member State in which the product is made available. In addition to labelling requirements, it also requires that this information be clearly understandable to the intended users ... What is the legislation in Europe for the languages to use on labels ... According to EU food (and water and beverage) legislation food labels must be given in a language that is understandable by consumers in the country in which you are selling your products. Therefore English will be good in the UK (and in Malta!) but not throughout Europe. This is the situation in EU countries but also the rest of the world. Product Labeling Regulations in the US, EU and Australia Here are few labeling requirements for imported products: a. The label must contain the identity of the prepackaged product in terms of its function or generic name. b. The label must contain the principal place of business and identity of the manufacturer or dealer. c.
EU: Language Requirements for Product Labels Labeling must include approval symbols from this institution and contain the following information about a product: - Clear identification of the products - Net quantity - Name and full address of producer or the importer/distributor - Country of Origin - Consumption expiry date - Recommended storage temperature EU - Labelling Requirements | CE Intelligence The use of language on labels has been the subject of a Commission Communication, which points out that labelling of foodstuffs for sale to the final consumer must be in an easily understandable language which is generally interpreted to mean the language of the country of marketing (European Commission ,2010). EU Labeling - Your Consultant on European Regulation Only once the EU compliance process has been almost completed (including the safety tests, safety assessment) will a product's label be verified and the requirements applied in reference to the specifics of the product. Scope & Classification. The Regulation: EC 1223/2009. EU Responsible Person. Product Information File. European Union Product Labeling Requirements: A Complete Guide Below follows criteria for Textiles and Furniture: Textiles 1. The product shall not contain lead-based pigments. 2. Manufacturers shall perform colorfastness, washing, wet rubbing, dry rubbing tests on dyed yarn, final fabrics, or final products. 3. Manufactured elastane shall not contain organotin compounds Furniture 1.
Official Language in EU required for Medical Device Labeling Medical Device Label - Language: EU Medical Device Regulations: 2: May 11, 2022: L: Language of quality system in case of FDA inspection: US Food and Drug Administration (FDA) 1: Feb 3, 2022: B: Software as a Medical Device - Language Requirements: EU Medical Device Regulations: 6: Jan 21, 2022: Q: World-wide language requirements: Other ...




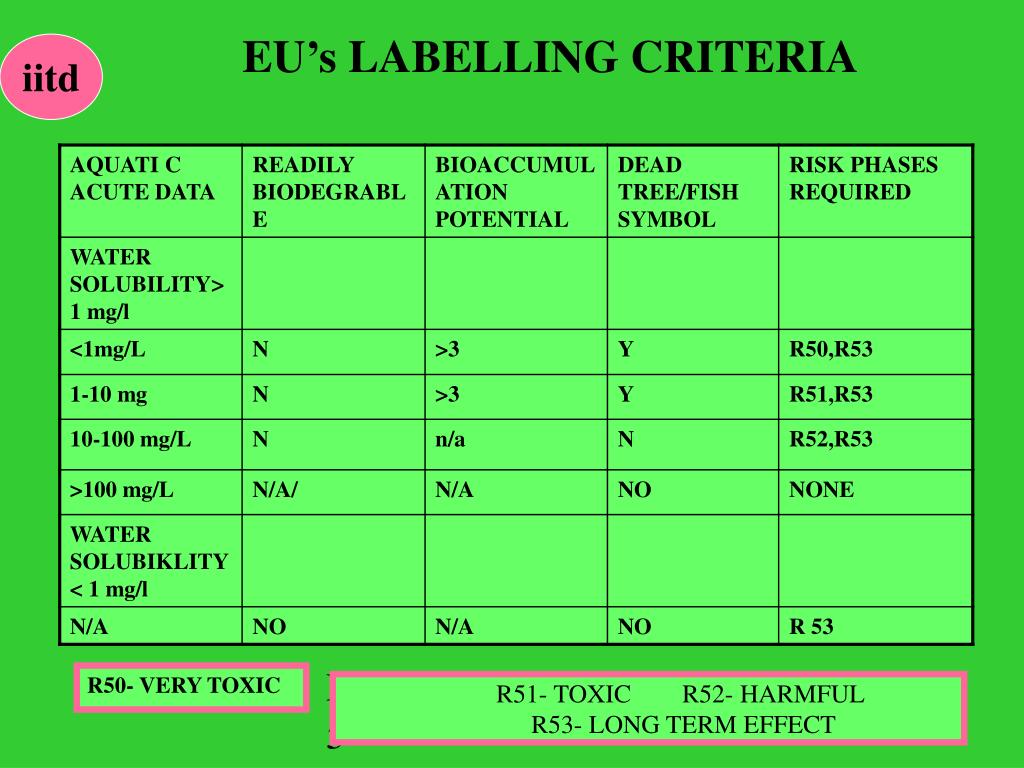
Post a Comment for "40 eu language requirements for product labels"