38 medication labels must include
› resources › safety-enhancements-everySafety Enhancements Every Hospital Must Consider in Wake of ... Place auxiliary labels on all storage locations and/or ADC pockets/ drawers/lids that contain neuromuscular blockers that clearly warn that respiratory paralysis will occur, and ventilation is required (e.g., “WARNING: CAUSES RESPIRATORY ARREST—PATIENT MUST BE VENTILATED”). The warning should be visible when ADC pockets/drawers/lids are open. How to read prescription drug labels - BeMedwise Whenever you are prescribed a medication, you should read and follow the information in the medication's "label" in order to ensure your safety. All prescription medicine containers include information on the label including the patient's name, the name of the medicine, dosage and instructions on how often to take the medicine.
PDF Chapter 20 Labeling Medications and Expiration Dating someone other than person administering, must also include patient name and location, directions for use, and auxiliary labels c. Other labeling considerations: ... NPSG.03.04.01: Label all medications, medication containers, and other solutions on and off the sterile field in perioperative and other procedural settings
Medication labels must include
The Over-the-Counter Medicine Label: Take a Look | FDA All nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an example of what the OTC... FDA says opioid labels must include information about naloxone - STAT By Lev Facher. Reprints. Scott Olson/Getty Images. W ASHINGTON — The Food and Drug Administration announced Thursday that it would require drug manufacturers to include information about ... Safety Enhancements Every Hospital Must Consider in Wake of … Include IV moderate sedation agents on high-alert medication lists. Include medications commonly used for moderate sedation (e.g., IV midazolam) on the hospital’s list of high-alert medications and implement risk-reduction strategies to …
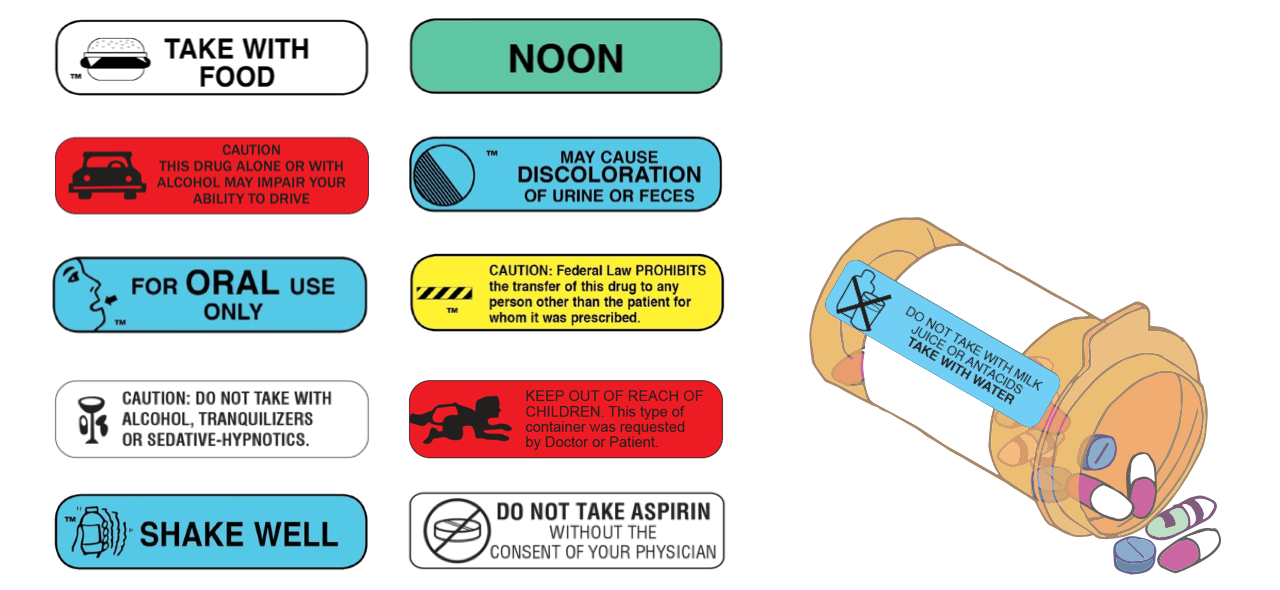
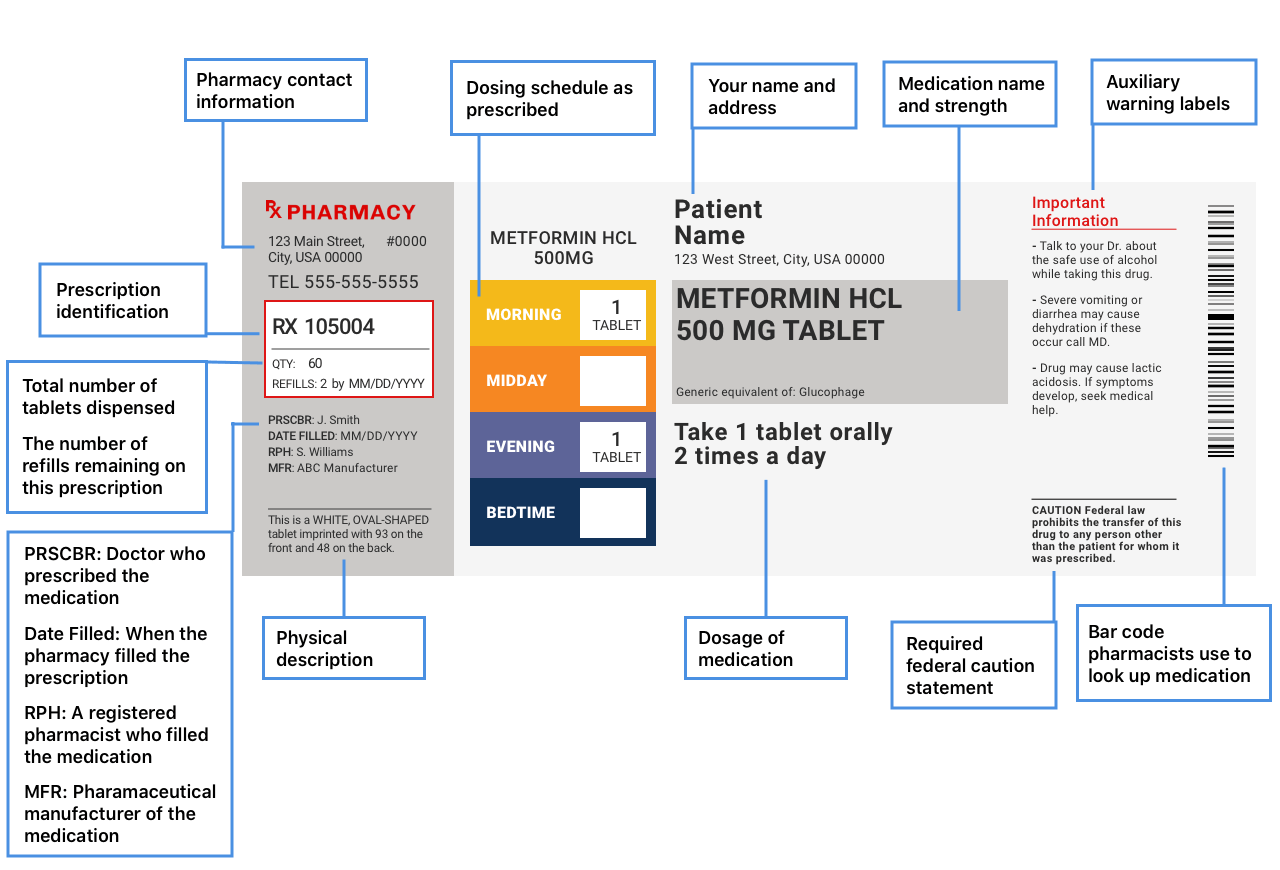
Medication labels must include. NCBOP - Pharmacist FAQs A. The following information must be on every prescription label: 1. Name and address of the dispensing pharmacy. 2. Serial number of the prescription. 3. Date of the prescription. 4. Name of the prescriber. 5. Name of the patient. 6. Name and strength of the drug. 7. How to Read Over-the-Counter and Prescription Drug Labels - Drugwatch.com Some labels include a seventh section with a phone number to call if you have questions or comments. The Drug Facts label for the over-the-counter drug acetaminophen, known by the brand name Tylenol, includes information about ingredients, uses, warnings and directions. Active Ingredient and Purpose. A Primer on Pharmaceutical Label Types and Requirements This FDA approved patient labeling typically includes medication guides and instructions for use. Whether or not a given medication must include a medication guide is determined by FDA regulations (usually, if they pose a significant health concern). PDF Labeling on the Sterile Field: Improve Patient Safety and Ensure Joint ... Labeling must include: Name of medication or solution, strength, date, and time Label one item at a time. Single items must also be labeled.
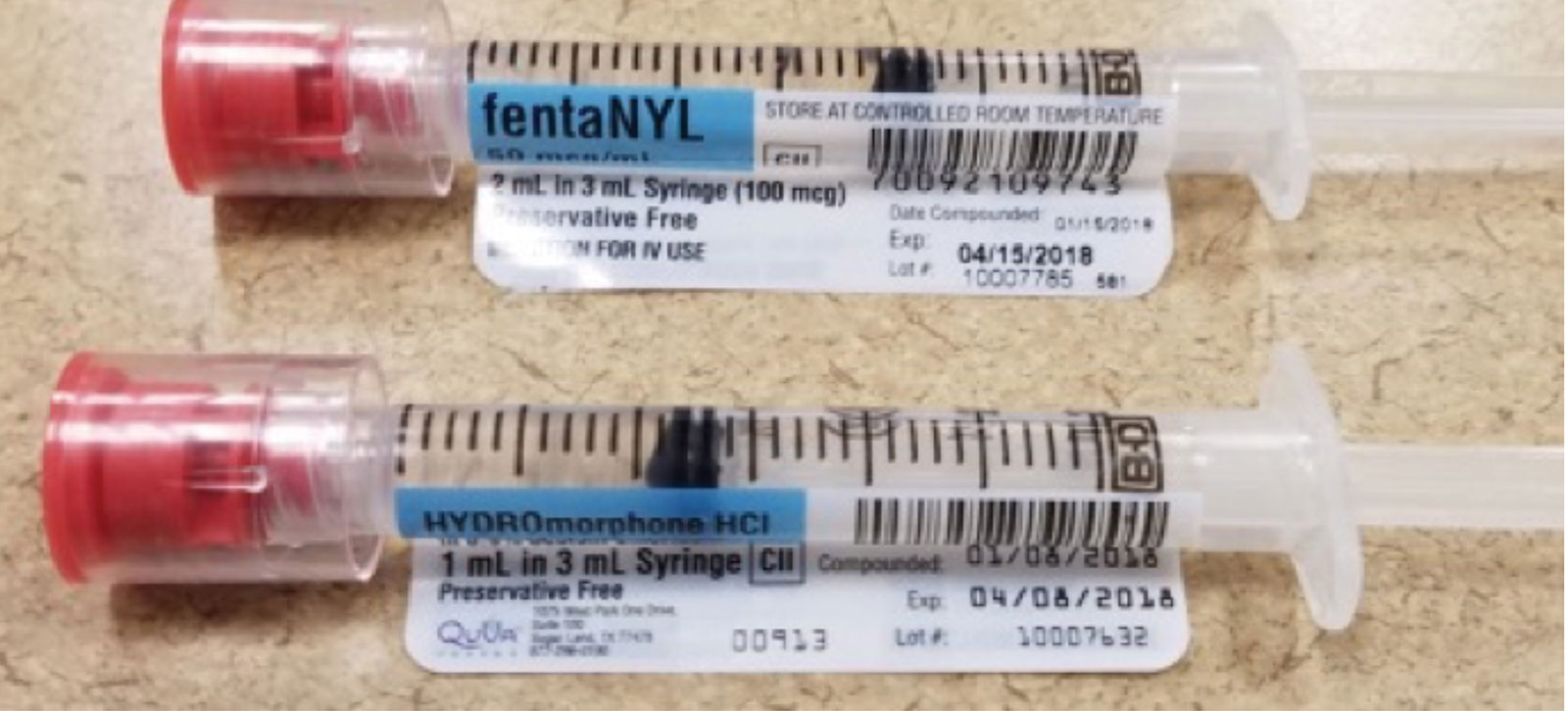
The Importance of Medication Administration: 5 Ways to … 13.3.2019 · Misreading medication names that look similar is a common mistake, particularly in hospitals, and particularly when orders are communicated verbally. To reduce the risk of these errors, use strategies such as: Use “Tall Man Lettering" - which helps distinguish similar drug names; Add warning labels that alert staff to LASA drugs What Is a Drug Label? | The Motley Fool Drug labels include instructions, ingredients, and a lot more information. Here's what you need to know from a healthcare investor's standpoint. A drug label refers to all the printed information ... Allergen labelling for food manufacturers | Food Standards … 8.9.2022 · If there is a risk of a food product being affected by allergen cross-contamination, the label should include one of the following statements: may contain X; not suitable for someone with X allergy; Precautionary allergen labelling should only be … Medication Administration Safety - Patient Safety and Quality There is a large and growing body of research addressing medication safety in health care. This literature covers the extent of the problem of medication errors and adverse drug events, the phases of the medication-use process vulnerable to error, and the threats all of this poses for patients. As this body of literature is evaluated, the fact that there are crucial areas about which …
Chapter 5: Prescriptions and Labels Flashcards | Quizlet Drug Labels Regulated by the Food and Drug Administration (FDA), which determines what needs to be on the label Dispensing pharmacist's label must include: Pharmacy name, address, and phone number Dispensing date Dispensing date may differ from the date on the prescription. Rx number, which identifies this unique prescription in the computer system How to Label Prescription Medication for Veterinary Patients A label should include the following components: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's... OTC Labeling Requirements - FindLaw (For example, drug products marketed under the Topical Antifungal Drug Products Monograph 2 should list their active ingredient's purpose as "Antifungal."). Below the "Drug Facts" section, a "Uses" section will list the approved or monograph indications for the drug. The next section of the label is "Warnings." Over the Counter (OTC) Drug Labels - Poison All OTC drug labels include "Drug Facts", the who, what, how, when, and why of that medicine. The Drug Facts tell you what you need to know to give the right drug, in the rightdose, to the right person, at the right time, in the right way, and for the right purpose.
› us › basicsPsychopharmacology | Psychology Today Psychopharmacology: #N# What Is Psychopharmacology?#N# #N#
› business-guidance › allergen-labellingAllergen labelling for food manufacturers Allergy related product withdrawals or recalls are often caused by incorrect packaging or labelling. Ensure that the correct labels are applied to products and any outer packaging. Packaging should be removed and destroyed at the end of a production run. This includes any that may be within the wrapping machine.
Basic Medication Administration 23.2.2009 · PRN medication must be documented. Each dose of medication must be recorded on the individual’s medication sheet, and the DSP should assure that a.m. or p.m. is noted too. To prevent errors always check for the last time a PRN medication was given before dispensing, and follow all individual medical protocols for that medication.
Medication Administration Flashcards | Quizlet Study with Quizlet and memorize flashcards containing terms like The nurse is administering medications to the client. What does the nurse explain to the client who asks about the checks of medication administration? Select all that apply., A nurse has administered a pain medication to the client. What should the nurse do next?, The client overhears the nurse reviewing the rights …
What's on a prescription label? - Knowledge is the best medicine It uniquely identifies all drug products sold in a dosage form in Canada and is located on the label of prescription and over-the-counter drug products that have been evaluated and authorized for sale in Canada. A DIN uniquely identifies the following product characteristics: manufacturer; product name; active ingredient (s); strength (s) of ...
› - › mediaPreventing Medication Errors in Hospitals - ASHP The organization must have a comprehensive program that includes a medication safety leader, key elements in place to provide the structure for safe medication practices, and a successful strategic plan. 10 Key supporting elements include a culture of safety built on principles of just culture that is
Guidance Document: Labelling of Pharmaceutical Drugs for Human Use (1.4) When the composition of the drug varies from one lot to another, the outer label must include a reference to all non-medicinal ingredient alternatives that may be present in the drug, ... Claims on drug product labels that include market share, sale, consumer and patient use/ choice, or preference must be supported by adequate studies ...
US Food and Drug Administration's Requirements on Content and Format ... The regulation required that drug labels include information about the frequency and severity of adverse reactions to drugs, as well as the organs affected. It provided a fill-in-the-blank sentence with space for the name of a drug, the reported reactions, and the likelihood of a consumer experiencing an adverse reaction.
FDA Says Drug Labels Must Include Clear Guidance for ... - Healthline Starting June 30, new drug labels will have categories for "Pregnancy," "Lactation," and "Females and Males of Reproductive Potential." "Pregnancy" will include information on ...
Guidelines for Labeling Pharmaceutical & Healthcare Products Several important things to include on a pharmaceutical or healthcare product label: 3. Formatting Labels for FDA Approval. Your labels must be designed in the appropriate FDA format for your product's classification like OTC medications, oral contraceptives, combination products, etc. Click here for a list of labeling guides relating to drugs.
Safe Labeling Helps Prevent OR Medication Errors - OR Today Label information must include a medication's name and strength as well as amount when medications are mixed (as with antibiotic irrigations, tumescent and heparin solutions, and epinephrine). The unit of measure — percent, grams, milliliters, or units — must be recorded along with the date the medication is prepared.
Preventing Medication Errors in Hospitals - American Society of … Safe medication practices begin with placing medication safety as anorganizational and departmental priority, and implementing a system that will support these practices. The organization must have a comprehensive program that includes a medication safety leader, key elements in place to provide the structure for safe medication practices, and a
FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,...
Medication package insert - Wikipedia A package insert is a document included in the package of a medication that provides information about that drug and its use. For prescription medications, the insert is technical, providing information for medical professionals about how to prescribe the drug. Package inserts for prescription drugs often include a separate document called a "patient package insert" with …
Barcode Medication Administration: Lessons Learned from an … Introduction. Medication errors are a serious public health threat. According to a landmark 1999 Institute of Medicine report, between 44,000 and 98,000 Americans die annually due to medical mistakes. 1 As part of its ongoing efforts to improve patient safety, the U.S. Food and Drug Administration (FDA) ruled on April 4, 2004, to make barcodes mandatory on the labels of …
en.wikipedia.org › wiki › Medication_package_insertMedication package insert - Wikipedia In the United States, the Food and Drug Administration (FDA) determines the requirements for patient package inserts. In the United States, the FDA will occasionally issue revisions to previously approved package inserts, in much the same way as an auto manufacturer will issue recalls upon discovering a problem with a certain car.
› books › NBK2656Chapter 37 Medication Administration Safety - NCBI Bookshelf Threats to medication safety include miscommunication among health care providers, drug information that is not accessible or up to date, confusing directions, poor technique, inadequate patient information, lack of drug knowledge, incomplete patient medication history, lack of redundant safety checks, lack of evidence-based protocols, and staff assuming roles for which they are not prepared.
Psychopharmacology | Psychology Today Psychopharmacology: #N# What Is Psychopharmacology?#N# #N#
A Guide To Veterinary Prescription Label Requirements What Is Required On A Veterinary Prescription Label As shown in the above example, the actual container must include the following information: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's last name
Pharmacology Chapter 5 (Prescriptions and Labels) - Quizlet Every prescription must include the following:-DATE-Pysicians name, contact info, and DEA number-Patients name, address, and DOB-INSCRIPTION -SIGNATURE ... what other medications include medication labels. over-the-counter (OTC) drugs. OTC labels contain instructions on drug use based on _ and _ age
4. Documenting Medications (MAR). | Aplmed Academy Each medication must be documented at the time of administration. For example, if eight medications are administered the QMAP must initial the MAR eight times indicating that each medication has been administered, refused or unavailable. New order: transcribe new medications on the MAR.
Pharmaceutical Labeling 101: FDA Regulations Guide These include drugs like analgesics, anti-inflammatory agents, antibacterial, anticonvulsants, and others. The substance is used in the diagnosis, mitigation, cure, treatment, or prevention of diseases. This category also includes supplements. The substance is a component of medication but not a part of a medical device.
Pharmaceutical Labeling: Requirements & Guidelines - CTM Labeling Systems To meet today's FDA regulations, labeling information on drugs must include the following in this order: - Product Name - Drug Facts Table - Active Ingredients - Purpose and Use - Warnings - Directions - Allergic Reactions - Inactive Ingredients
› centrain › Training_UnitsBasic Medication Administration - CMHCM Feb 23, 2009 · PRN medication must be documented. Each dose of medication must be recorded on the individual’s medication sheet, and the DSP should assure that a.m. or p.m. is noted too. To prevent errors always check for the last time a PRN medication was given before dispensing, and follow all individual medical protocols for that medication.
5 Things to Look For in a Prescription Medication Label - Epler Health 5 Things to Look For in a Prescription Medication Label. According to a 2006 Institute of Medicine report, about 1.5 million preventable medication errors are made each year. Prescription drugs are a double-edge sword. They can treat and manage diseases, making it possible to lead a better life.
Medicines: packaging, labelling and patient information leaflets Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional warning statements must be included on the packaging of...
Drug labeling, Information about Drug labeling - FAQs Each product must contain a label with "Supplement Facts" in bold letters onthe front panel. This is the manufacturer's opportunity to identify the product. Below "Supplement Facts," the panel must state the serving size. This isdetermined by the manufacturer with no input from the FDA.
Safety Enhancements Every Hospital Must Consider in Wake of … Include IV moderate sedation agents on high-alert medication lists. Include medications commonly used for moderate sedation (e.g., IV midazolam) on the hospital’s list of high-alert medications and implement risk-reduction strategies to …
FDA says opioid labels must include information about naloxone - STAT By Lev Facher. Reprints. Scott Olson/Getty Images. W ASHINGTON — The Food and Drug Administration announced Thursday that it would require drug manufacturers to include information about ...
The Over-the-Counter Medicine Label: Take a Look | FDA All nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an example of what the OTC...

























Post a Comment for "38 medication labels must include"